AVAREF Statutory Bodies
The African Medicines Regulatory Harmonization (AMRH) Initiative
In 2019, the AVAREF Assembly adopted a new governance structure to align with the African Medicines Regulatory Harmonization (AMRH) Initiative. This new structure transformed AVAREF’s Technical Coordination Committee into a Continental Working Group, and the AVAREF Steering Committee was re-established as an Advisory Committee to provide strategic guidance for the Working Group Committees.
AVAREF is in the process of implementing this new governance structure. A detailed description of these bodies is provided below.
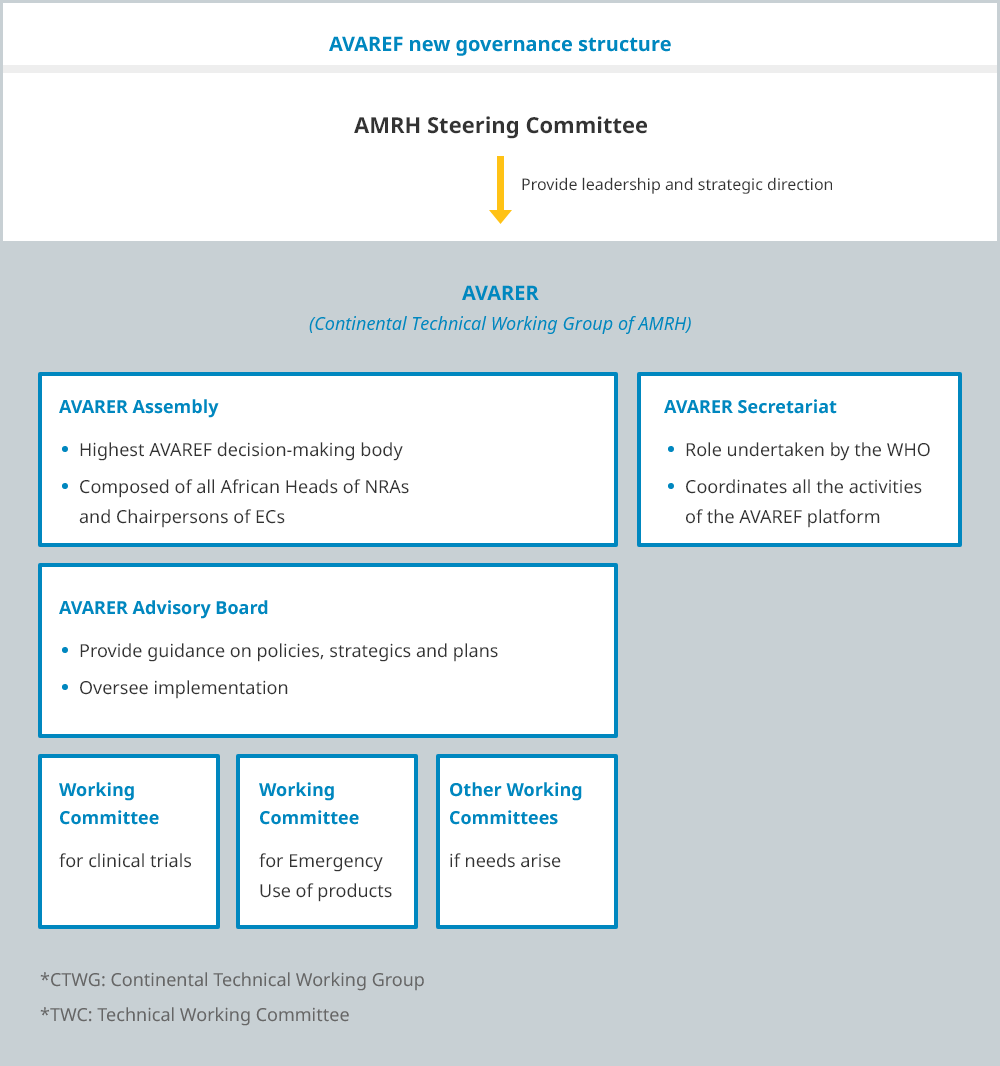
Four bodies manage AVAREF’s strategic and operational functions:
- The Assembly is the overarching body of AVAREF and is composed of all African Heads of National Regulatory Authorities (NRAs) and Chairpersons of National Ethics Committees (ECs) or their representatives.
The Assembly is the highest AVAREF decision-making body. A special session is convened at the annually held African Medicines Regulators Conference (AMRC) that is attended by all heads of agencies, who also form the Assembly. - The Steering Committee comprises member representatives from the eight Regional Economic Communities (RECs) in the African Union, and defines AVAREF policies, strategies and plans, as well as oversees their implementation by AVAREF members.
- The Technical Coordination Committee, which works closely with the AVAREF Secretariat and the Steering Committee, provides advice and guidance on scientific and technical matters.
AVAREF Statutory Bodies: Leadership and Members
Chair and Vice-Chair of the Steering Committee/Advisory Committee
The Chair and Vice-Chair of the Steering Committee (or “Advisory Committee”) are responsible for operationalizing AVAREF guidelines and processes, as well as supporting AVAREF’s day-to-day activities, which they do in collaboration with the AMRH Joint Secretariat. The Assembly, which is made up of all Heads of National Regulatory Agencies and Chairpersons of National Ethics Committees in Africa elects the Chair and Vice-Chair from among candidates nominated by the Steering Committee. One is drawn from a National Regulatory Authority, and the other from a National Ethics Committee. They serve a maximum 3-year term and are ineligible for immediate re-election.
The Steering Committee/Advisory Committee
The Steering or Advisory Committee, which consists of 16 representatives, are drawn from the eight Regional Economic Communities (RECs) in the African Union (i.e., the Economic Community of West African States (ECOWAS), the East African Community (EAC), the Southern African Development Community (SADC), the Inter-Governmental Authority on Development (IGAD), the Arab Maghreb Union (AMU), the Common Market for Eastern and Southern Africa (COMESA), the Community of Sahel-Saharan States (CENSAD), and the Economic Community of Central African States (ECCAS). The Committee is selected through nominations and voting of members, and provides direction, oversight and accountability for AVAREF by presenting annual reports, work plans and strategies to the Assembly for endorsement and ratification. It also determines the host and dates for the annual AVAREF Assembly and quarterly Steering Committee meetings. Finally, the Steering/Advisory Committee establishes and oversees the efficiency and output of AVAREF’s working groups and task forces. Committee members serve a maximum 3-year term and are ineligible for immediate re-election.
Currently, the members on the Steering Committee are drawn from the Democratic Republic of the Congo (DRC), Equatorial Guinea, Ethiopia, Ghana, Kenya, Mali, Mozambique, Senegal, South Africa, South Sudan, Tanzania and Zambia. Plans are underway to align the Statutory bodies with the AMRH governance model.
The current AVAREF Steering Committee Chairperson is Mrs. Delese Mimi Darko, CEO, Ghana Food and Drugs Authority, Ghana.
Current Steering Committee (SC) Members:
- Drabo Maxime, Comité d’éthique, Burkina Faso
- Eric Karikari-Boateng, Ghana Food & Drugs Authority, Ghana
- Priscilla Nyambayo, Medicines Control Authority, Zimbabwe
- Edward Abwao, Pharmacy and Poison Board, Kenya
- Winfred Badanga, Uganda National Council for Science & Technology, Uganda
- Marie Claire Okomo Assoumou, Ethics Committee, Cameroon
- Okouyi Noakissa, Direction du Médicament et de la Pharmacie, Gabon
- Collins Mitambo, National Ethics Committee, Malawi
The AVAREF Secretariat
Working collaboratively with the Steering Committee and the Technical Coordination Committee, the AVAREF Secretariat is responsible for overseeing the day-to-day operations of AVAREF and coordinating the activities of its members, as well as facilitating meetings of the Committees and Task Force/Sub-Working groups. The roles of the Secretariat also include communication, fundraising, coordinating the development of processes and guidance documents, and disseminating AVAREF regulatory tools. The Secretariat comprises WHO technical officers. It engages in the following key technical roles and functions:
- Regulatory Strengthening
- Regulatory Harmonization
- Clinical Trial Oversight & Monitoring
Regulatory Strengthening
AVAREF was established to address the challenges confronting ethics and regulatory authorities and to strengthen ethics and regulatory oversight of clinical trials using a network approach. AVAREF’s work is based on strengthening capacity through reliance and cooperation, while recognizing national ownership, leadership, and transparency. AVAREF uses harmonization and reliance as pillars for capacity building, ensuring that there is collaboration between NRAs and national ECs in and among countries by promoting joint reviews, work-sharing and use of expertise of all involved, including key partners.
Regulatory Harmonization
As an effort to improve clinical trial review practices, AVAREF Member States agreed to a maximum timeline of 60 calendar days for processing and clearance of clinical trial applications. The AVAREF Assembly, composed of heads of NRAs and ECs of Member States, met in Accra, Ghana, in November 2017 and endorsed this timeline. The Secretariat has established that not all Member States are consistently meeting these timelines due to their different capabilities for assessing clinical trial applications, stressing the need for further harmonization. Member States have also recognized the importance of harmonization to address gaps in their capacities and processes.
Clinical Trial Oversight & Monitoring
As an effort to improve clinical trial oversight practices and capacity in reviewing technical documents of clinical trial applications, two AVAREF technical working groups – the Technical Working Group on Inspection of Good Clinical Practices (GCP) and the Clinical Trials Working Group (CTWG) – developed standardized templates and guides for GCP inspection, submission and assessment of clinical trial applications, and monitoring of clinical trials in the African region.


