Overview: The AVAREF Joint Review Process
AVAREF offers a one-stop end-to-end process for clinical trial application (CTA) and helps improve the regulatory landscape in Africa. Through joint reviews, ethics committees and regulators of participating countries review and exchange thoughts together and speed up the CTA review process. Members of AVAREF agreed upon the harmonized regulatory process to enhance efficiency and improve transparency. Ethics committees and regulators of member states builds strong partnership by participating joint reviews. AVAREF also provides support to member states in building institutional and technical capacity to meet demand in Africa.
Once an applicant (product developer) submits their expression of interest to the AVAREF Secretariat, a preliminary meeting can be scheduled to discuss a type of joint review pathway and to agree on next steps for joint reviews. National regulatory authorities and national ethics committees and institutional review boards are invited to participate in joint review process and agree on timelines depending on the types of joint review pathway. AVAREF Secretariat supports in the coordination of joint reviews by facilitating meetings and ensuring the timelines are met.
Joint reviews are intended to enhance the quality of the reviews of an application submitted to multiple countries, optimize review timelines for such applications, serve as a platform to allow regulators and ECs to exchange and validate their findings with peers and also act as a capacity-building tool. Joint reviews enable NRAs and ECs to collectively prepare a consolidated list of questions for the sponsor and to discuss directly with manufacturer/sponsor the candidate product, trial design, safety and other aspects of the proposed trial.
AVAREF Joint Review Pathways
With the recent addition of an emergency joint review process, there are now three distinct options for joint reviews under the umbrella of support provided by the AVAREF platform to Member States and sponsors.
There are three possibilities for an applicant who wishes to utilize the AVAREF platform for review of a clinical trial application in more than one country within the continent.
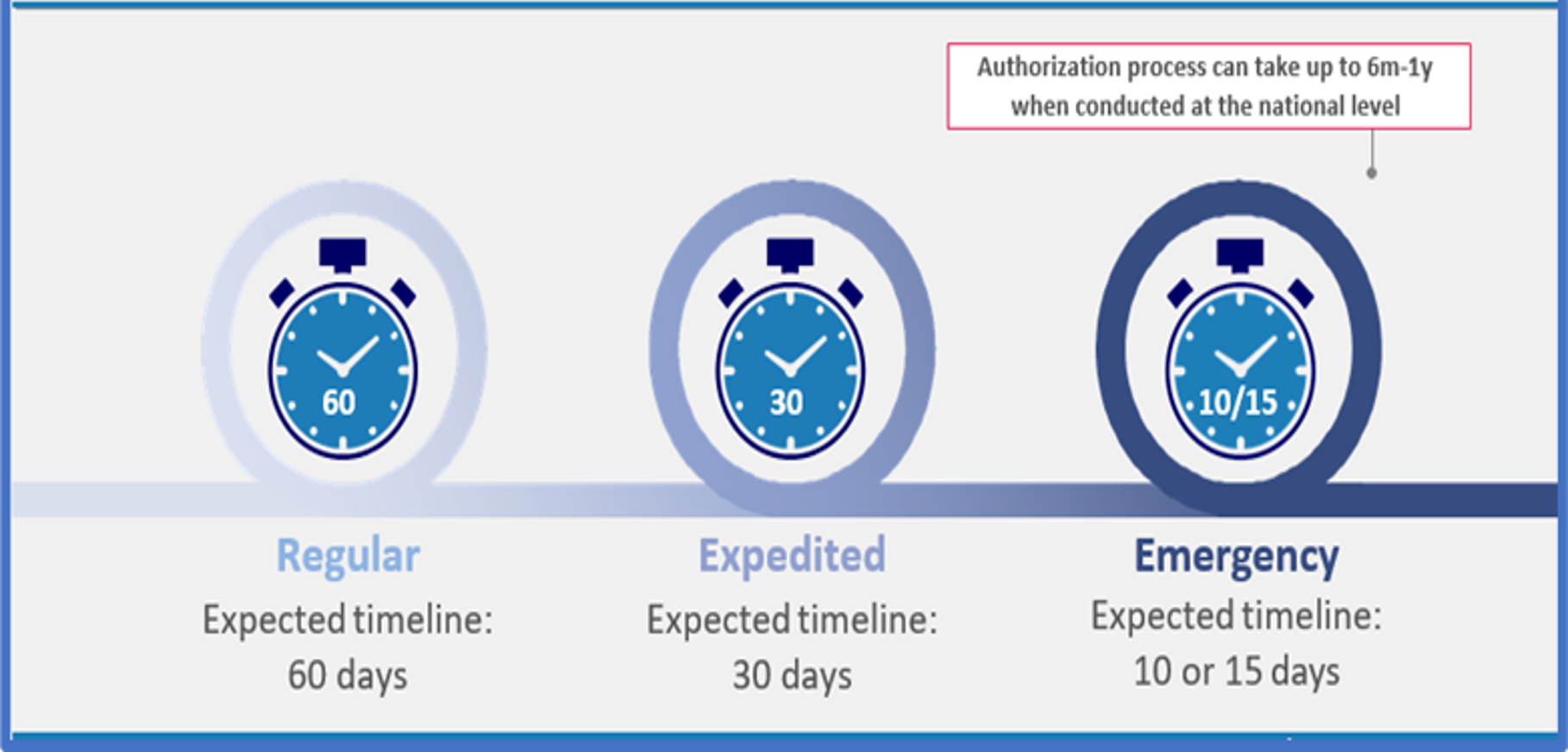
Timelines for 3 different AVAREF Joint Review Pathways
There are joint review specific criteria for elements of the application and the investigational product which would in principle, qualify a clinical trial application for review using one of the three options. The timelines differ significantly, as represented in the figure ‘Timelines for AVAREF Joint Reviews’. When applicant and the Secretariat meet for preliminary meeting, the type of joint review pathway can be reviewed and confirmed.
Information on eligibility criteria, initiating the joint review process by submitting a notice of intent to the AVAREF Secretariat, and details of the steps in the various joint review processes are described in the AVAREF Joint Review Guideline, which contains details for the Regular and Expedited Joint Review processes, and the AVAREF Strategy and Guidance for Emergency Preparedness, which contains the details for Emergency Joint Reviews.
Between March and May 2020, the AVAREF Technical Coordinating and Steering Committees, supported by the AVAREF Secretariat, developed a critical strategy and guidance document, making possible the organized conduct of a monumental emergency joint review in subsequent weeks.
In addition to joint reviews, AVAREF has options for the following other types of clinical trial reviews: regular, expedited, and emergency joint reviews. When a participating regulator or ethics committee requests, the Secretariat supports to facilitate assisted review by inviting regulators or ethics committee members from other countries to joint review meetings.

Regular joint review
Regular joint review pathway is used for joint review of clinical trial application of vaccines or therapeutic medicines which do not fall in the category of expedited or emergency joint review process. To be considered for joint review, a candidate medical product of high public health value to countries on the African continent will be considered based on one or more of the following criteria:
- Addresses a neglected tropical disease or other highly prevalent and serious disease (e.g., non-communicable disease – NCD) on the continent
- Addresses an unmet medical need or a significant improvement over available intervention
- Involves a novel technology
- Product that addresses a disease for which the Director General of the World Health Organization has declared a Public Health Emergency of International Concern (PHEIC)
- Responds to request from one or more countries for assistance
Other criteria may be considered based on the needs of the countries.

Expedited joint review
AVAREF expedited review process can be used both for joint reviews and assisted reviews to support member states to expedite the ethical and regulatory review processes without sacrificing stringency of technical reviews under emergency situations. The current AVAREF guidelines recommends to complete the entire review processes within 25 working days through joint reviews which involves both ethical and regulatory reviews at the same time rather than potential parallel reviews.

Emergency joint review
The AVAREF Joint Review Guideline describes the expected timelines for various steps of the joint review and expedited joint review process, however the timelines are still considered to be too long for use in emergency joint reviews during a pandemic situation. For the emergency joint review process to be meaningful, it should be conducted within an overall timeline of 10 working days for processing of CTAs where the product is already registered for other indication(s), and 15 working days for novel products. These timelines are for the entire process from receipt of CTA to the final decision at country level, with exception of clock stops, including both ethics and regulatory review. Once the AVAREF Joint Reviews are completed, the remaining administrative procedures need to be followed at country level in order to enact for provisions of authority to enable the initiation of clinical trials in participating countries.
How Does a Joint Review Work?
Sometimes clinical trials are planned in more than one country and at several sites in these countries for the same product. For such a multicenter, multi-country clinical trial, clinical trial applications (CTAs) will have to be submitted to individual NRAs and ECs, often in different formats as defined by each country. Reviews will then be conducted individually and at different times before final outcomes are communicated individually to the sponsor. Furthermore, many of the questions submitted by countries will be similar in nature. To optimize the review of multi-country clinical trial applications, promote harmonization of regulatory requirements, practice and processes across countries, as well as build capacity for more efficient oversight, WHO introduced the concept of joint reviews in 2006.
Joint reviews are intended to enhance the quality of the reviews of an application submitted to multiple countries, optimize review timelines for such applications, serve as a platform to allow regulators and ECs to exchange and validate their findings with peers and also act as a capacity-building tool. Joint reviews enable NRAs and ECs to collectively prepare a consolidated list of questions for the sponsor and to discuss directly with manufacturer/sponsor the candidate product, trial design, safety and other aspects of the proposed trial.
The graphic below depicts the four points at which stakeholders collaborate during the joint review process.

Benefits
The joint review process allows simultaneous review of a clinical trial application by all participating countries, with parallel review by responsible authorities in-country. Whereas a regular application to a single member state might take up to a year to process, joint reviews through the AVAREF platform take a maximum of 60 days—and fewer for expedited or emergency reviews.
In the event of pandemics/epidemics or other health emergencies, the AVAREF platform is well positioned to accelerate product development, clinical trials approval and access to life saving vaccines and medicines. The role of AVAREF extends beyond the conduct of joint reviews including engagement and provision of guidance to RECs, research consortia and individual countries with regards to emergency situations.
Who is Eligible?
AVAREF will consider a medical product for joint review if it meets one or more of the following criteria:
- It addresses a neglected tropical disease or a highly prevalent, serious non-communicable disease on the continent
- It addresses an unmet medical need or may result in a significant improvement over available interventions
- It involves a novel technology
- It addresses a disease for which the Director-General of the World Health Organization (WHO) has declared a public health emergency of international concern
AVAREF may consider other criteria as well, based on a Member States’ needs.
How does a country/sponsor initiate a joint review?
A proposal to initiate a joint review through AVAREF may come from a sponsor, a product development partner, an AVAREF member state, WHO, or another international organization. In all cases, the interested party should communicate their request to AVAREF at: afrgoavaref [at] who.int (afrgoavaref[at]who[dot]int) to request application forms and any other information required.
Where can a country/sponsor find more information about the joint review process?
The AVAREF Joint Review Guideline describes the regular and expedited joint review processes in detail. The AVAREF Strategy and Guidance for Emergency Preparedness document also describes the emergency joint review process in detail.


