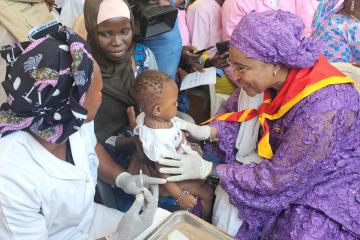
- Immunization through vaccination is the safest way to protect against disease. Vaccines protect us throughout life and at different ages, from birth into childhood, adolescence and old age.
- Without vaccines, we are at risk of serious illness and disability from diseases like measles, meningitis, pneumonia, tetanus and polio, among others. Many of these diseases can be life-threatening.
- When vaccine coverage rates drop, once-scarce deadly diseases – such as measles – can re-emerge. Universal immunization coverage is needed to help end vaccine-preventable diseases once and for all.
- Immunization is a low-cost, high-impact health intervention and a key driver of universal health coverage (UHC).
- Immunization currently prevents over 4 million deaths every year. In the African Region alone, around 800,000 lives are saved every year because of vaccines.
- Immunization is one of the most successful and cost-effective public health interventions: It is estimated that every $1 spent on childhood immunizations in Africa returns $44 in economic benefits. Investing in immunization keeps children healthy and safe from preventable diseases.
- Infants and young children are often at the greatest risk from diseases because their immune systems are not yet fully developed, and their bodies are less able to fight off infection. It is therefore very important that children are vaccinated against diseases at the recommended time.
- One in five children in Africa still do not receive all the necessary and basic vaccines. Every year, approximately 30 million African children fall sick from vaccine-preventable diseases, and half a million of them die as a result.
- Important progress has been made in vaccine research and development: The first vaccine to protect children against malaria was piloted in Ghana, Kenya and Malawi in 2019, while new vaccines to protect against Ebola are being used to help control the outbreak in the Democratic Republic of the Congo (DRC).
- In 2012, more than 100 cases of wild poliovirus were reported in the African Region – representing more than half of the global burden. No new cases have been reported since 2016, and in 2019 the African Region marked three years since the last confirmed case of wild poliovirus, a major milestone on the path towards eradication. The African Region is expected to be certified wild-polio-free in 2020.
- Meningitis A epidemics have been drastically reduced in Africa through immunization. Since the introduction of the meningitis A vaccine in Africa in 2010, mass vaccination campaigns have led to the control and near elimination of the deadly meningitis A disease in 26 African “meningitis belt” countries. The vaccine is now being integrated into routine national immunization programmes.
- If immunization efforts in African are not maintained, there is a risk to reverse progress made, leading to more than 2.4 million deaths and a negative economic impact of $59 billion over the next decade. Strengthening routine immunization saves lives and is a smart investment for the future.
Vaccine-preventable diseases (VPDs) refer to the range of diseases that can be prevented and controlled by vaccination. The burden of VPDs and associated outbreaks remain a major threat to people across Africa. Every year, more than 30 million African children under the age of five suffer from VPDs; of these, more than half a million die due to limited access to immunization services – representing 58% of global deaths from VPDs.
Recurrent VPD outbreaks persist in many countries in the African Region. VPD-related outbreaks tend to appear in areas where the immunization coverage rate is low, therefore enabling transmission.
Major VPDs affecting the African Region include:
- Cholera
- Ebola Virus Disease
- Hepatitis
- Human papillomavirus (HPV)
- Influenza
- Malaria
- Maternal and neonatal tetanus
- Measles
- Meningococcal Meningitis
- Pneumonia
- Polio
- Rotavirus
- Rubella
- Tetanus
- Typhoid Fever
- Yellow Fever
Four major vaccine-preventable diseases – rotavirus, pneumococcal diseases, measles and rubella – collectively cost the African Region $13 billion each year, due to loss of productivity as a result of premature death ($10 billion) and prolonged sickness ($2 billion), hospitalizations ($260 million) and outpatient visits ($73 million).
Sustained control, elimination and eradication of key VPDs is vital to end preventable deaths and protect the health and wellbeing of populations across the African Region. WHO works closely with countries and partners to improve immunization coverage and prevent and control VPDs, as part of broader efforts to strengthen primary health care and help countries move towards UHC. WHO supports all countries in the African Region by establishing norms and standards, developing evidence-based policies and guidance, monitoring VPD situations, contributing to health service delivery in emergency situations, and advocating with governments and partners.
Disease surveillance involves the systematic collection, analysis, interpretation, and dissemination of information regarding the occurrence of diseases in defined populations for public health action. Vaccine-preventable disease (VPD) surveillance refers to surveillance efforts primarily focusing on diseases that can be prevented or controlled by vaccination.
VPD surveillance is critical to protect populations from disease – it is the foundation of disease control strategies and the most effective way to detect and respond early to outbreaks, in order to mitigate their impact on national security, the local economy and public health systems. VPD surveillance data is used to guide effective response to outbreaks, assess progress in attaining set immunization targets, determine barriers to access, and identify gaps. High quality surveillance is also important to inform decision-making by policy makers. Strengthening surveillance systems and laboratory networks is critical to accelerate efforts towards achieving universal immunization and protecting populations from disease.
All countries in the African region have committed to universal immunization coverage and high-quality surveillance, but challenges remain in achieving targets outlined in the Regional Strategic Plan for Immunization (RSPI). Countries in the African Region still face major challenges in both the strategic planning and operation of their surveillance systems, including fragmentation, lack of public resources, difficulties in leveraging community-based surveillance, inadequate surveillance of some diseases, challenges with staff retention and training, and transportation issues.
Over the past two decades, VPD surveillance in the African Region has been conducted in an integrated manner, supported heavily by funding from the Global Polio Eradication Initiative (GPEI). However, as the region approaches the official certification of polio eradication, significant resources for surveillance through the GPEI will ramp down, and eventually end by 2023. Similarly, most countries in Africa will transition out of receiving support from Gavi, the Vaccine Alliance, by 2030.
This imminent decrease in funding has the potential to significantly reverse progress made, particularly through the loss of surveillance support. By 2030, the complexity and demand for VPD surveillance is also expected to widen. Further investment is therefore critical to sustain levels of funding to support VPD surveillance and laboratory activities. In the coming years, African countries, partners and donors will need to urgently revitalize their disease surveillance systems and invest considerably in this area, to fill these gaps to ensure that hard-won progress is not reversed.
Over the years, people have increasingly expressed concerns about the potential risks associated with vaccines. An adverse event following immunization (AEFI) is any untoward medical occurrence which follows immunization, and which does not necessarily have a causal relationship with the usage of the vaccine. If not rapidly and effectively dealt with, these perceptions can undermine confidence in a vaccine and ultimately have dramatic consequences for immunization coverage and disease prevention.
To ensure safe use of vaccines through close monitoring of vaccines-associated adverse events in the region, the VPD Programme at the WHO Regional Office for Africa supports National Regulatory Authorities (NRAs) to strengthen the safety monitoring systems and build capacity to conduct prompt and scientific causality assessments.
In order to respond promptly, efficiently, and with scientific rigour to vaccine safety issues, the WHO Regional Office for Africa has established an African Advisory Committee on Vaccine Safety (AACVS). This committee provides independent expert advice on technical issues related to the safety, effectiveness and use of all vaccines, consistent with the Global Vaccine Action Plan (GVAP), the Regional Strategic Plan for Immunization (RSPI) and the Global Vaccine Safety Blueprint (GVSB).
Read more about:
The African Region has the highest incidence of mortality caused by infectious diseases, yet has limited capacity to manufacture affordable, essential vaccines. Challenges in scaling up vaccine research and development in the African Region include lack of government coordination, limited capacity of national committees (including national ethics and scientific review committees as well as national regulatory authorities), inadequate funding, and limited research skills and facilities.
Despite these challenges, improvements in scientific knowledge and development of new technologies – as well as the support and leadership of the African Vaccine Regulatory Forum (AVAREF) – have helped to accelerate vaccine development and clinical trials in the African Region. AVAREF works with national ethics committees and regulatory authorities to ensure timely regulatory evaluations and decision-making processes on clinical trial applications and to reduce the lengthy timelines for their review and approval. The forum has supported the development and/or review of multiple vaccines in the African Region, including vaccines against malaria, meningitis, rotavirus, pneumococcal pneumonia, cholera, HPV and Ebola.
Many clinical trials have been conducted and increasingly more are being planned in the African Region to evaluate the efficacy and safety of candidate vaccines. However, additional efforts are needed in this area to enhance regulatory oversight for the development and approval of new vaccines that meet quality, safety, and efficacy standards and build manufacturing capacity.
National Immunization Technical Advisory Groups (NITAGs) are multidisciplinary groups of national experts responsible for providing scientific recommendations to their respective ministries of health, to enable them to make independent, evidence-based policy and programme decisions related to immunization. The Global Vaccine Action Plan calls for all countries to establish or have access to a NITAG by 2020. As of 2018, 30 of the 47 countries in the African Region had established a NITAG, whereas 17 had not. Out of the 30 countries with NITAGs, 18 met all the WHO functionality criteria.
WHO places a high priority on the development of national decision-making process and capabilities, including the establishment and strengthening of functional, independent NITAGs. WHO supports countries in the African Region by providing technical guidance for the establishment/strengthening of a NITAG, as well as providing technical guidance, including global and regional policy recommendations, to the NITAG in formulating immunization policies and strategies.
The African Regional Immunization Technical Advisory Group (RITAG) is the principal advisory group to the WHO Regional Office for Africa to provide strategic guidance on vaccines and immunization. RITAG reports directly to the WHO Regional Director for Africa and advises the Regional Director on overall regional policies and strategies, ranging from vaccine and technology research and development, to delivery of immunization services and linkages between immunization and other health interventions. Its remit is not restricted to childhood immunization but extends to all vaccine-preventable diseases as well as all age groups. Members of the RITAG are appointed to serve for an initial term of three years, renewable once.
Additional resources:
- Report of the Regional Immunization Technical Advisory Group meeting, November 2020
- Report of the Regional Immunization Technical Advisory Group meeting, July 2020
- Report of the Regional Immunization Technical Advisory Group meeting, January 2019
- Report of the Regional Immunization Technical Advisory Group meeting, June 2018
- Report of the Regional Immunization Technical Advisory Group meeting, December 2017
- Report of the Regional Immunization Technical Advisory Group meeting, June 2017



 Immunization is one of the most impactful and cost-effective public health interventions available, averting over 4 million deaths every year. In addition to offering protection from preventable diseases, immunization also brings children and families into contact with health systems, providing an avenue for the delivery of other basic health services and laying the foundation for primary health care. As such, ensuring universal access to vaccines is a critical entry point for universal health coverage (UHC).
Immunization is one of the most impactful and cost-effective public health interventions available, averting over 4 million deaths every year. In addition to offering protection from preventable diseases, immunization also brings children and families into contact with health systems, providing an avenue for the delivery of other basic health services and laying the foundation for primary health care. As such, ensuring universal access to vaccines is a critical entry point for universal health coverage (UHC). On 31 January 2017, at the 28th African Union (AU) Summit, Heads of State from across Africa endorsed the
On 31 January 2017, at the 28th African Union (AU) Summit, Heads of State from across Africa endorsed the 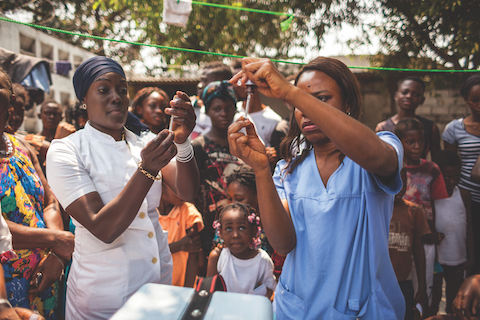 African Vaccination Week (AVW) is an annual event celebrated during the last week of April, with the aim of strengthening immunization programmes in the African Region by increasing awareness of the importance of every person’s need and right to be protected from vaccine-preventable diseases. The campaign, which is held in synchronization with the annual World Immunization Week, brings together all countries across the region to raise awareness of the benefits of vaccination. AVW’s overarching slogan is “Vaccinated communities, Healthy communities”. Each year, a theme is chosen to reflect regional public health priorities.
African Vaccination Week (AVW) is an annual event celebrated during the last week of April, with the aim of strengthening immunization programmes in the African Region by increasing awareness of the importance of every person’s need and right to be protected from vaccine-preventable diseases. The campaign, which is held in synchronization with the annual World Immunization Week, brings together all countries across the region to raise awareness of the benefits of vaccination. AVW’s overarching slogan is “Vaccinated communities, Healthy communities”. Each year, a theme is chosen to reflect regional public health priorities. In 2006, WHO created the African Vaccine Regulatory Forum (AVAREF) as an informal capacity-building platform aimed at improving the regulatory oversight of interventional clinical trials being conducted in Africa.
In 2006, WHO created the African Vaccine Regulatory Forum (AVAREF) as an informal capacity-building platform aimed at improving the regulatory oversight of interventional clinical trials being conducted in Africa.